a unique, patented
thermal diffusion coating technology

Galvanizing — or zinc coating — has been used for over a hundred years in a wide range of applications to protect steel from the ravages of corrosion. Greenkote® is an advanced, patented, thermal diffusion process that takes zinc-based corrosion resistant coatings to a whole new level of performance.
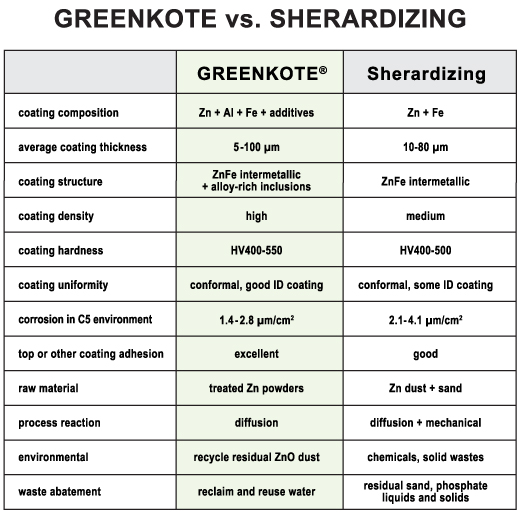
For corrosion protection, this is a technically superior replacement for zinc plating, hot dip coating, mechanical galvanizing, zinc flake dip-spin and various other competitive processes. In some cases, Greenkote can even replace stainless steel or chromium plating. Greenkote delivers exceptional protection against corrosion and also eliminates hydrogen embrittlement, provides superior adhesion and greater uniformity. It wears longer and is competitively priced — plus, it’s completely eco-friendly! Greenkote processes and coatings are totally free of toxics and pollutants, including heavy metals and Cr6+ and Cr3+.
 A unique thermal diffusion coating process — compliant with ASTM A1059/A1059M
A unique thermal diffusion coating process — compliant with ASTM A1059/A1059M
Greenkote corrosion resistant coatings are applied by a patented Thermo-Chemical Surface Modification (TCSM) batch process that is fully compliant with the ASTM A1059/A1059M specification, for cost-effective, high performance corrosion protection. (See the specification and news about it.) Greenkote utilizes a dry bulk powder formulation of zinc, aluminum and other proprietary ingredients that is thermo-diffused into the iron-based metal substrate surface of finished metal parts. The Greenkote coating forms in two basic phases…
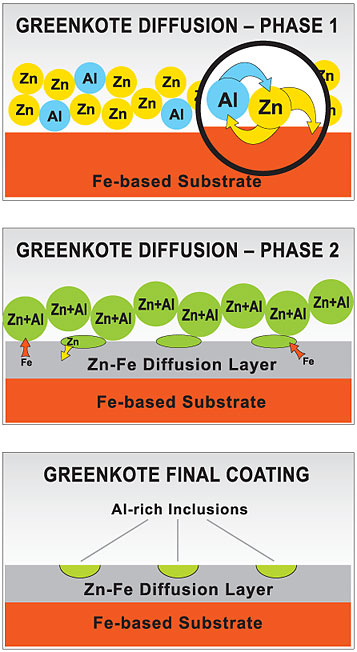
Greenkote process technology — Phase One:
Starting parts must be free of oil, oxides and other debris and are typically cleaned immediately prior to the coating process using traditional cleaning technologies.
The clean parts are placed in a large metal retort along with the proprietary Greenkote powder containing the key ingredients of zinc (Zn) and aluminum (Al). The retort is sealed and commences to rotate while its temperature is ramped through the required temperature profile.
With increased temperature the Zn diffuses into the Al powder and also into the iron (Fe) surface of the substrate. At the same time Al diffuses into the Zn powder. These processes initiate a conformal Zn-Fe diffusion layer and also a Zn-Al powder with a lower melting temperature.
Greenkote process technology — Phase Two:
Melted grains of the Zn-Al powder are absorbed onto the Zn-Fe diffusion layer and begin to locally react to create a ternary Al-Zn-Fe intermetallic. The increased Fe content raises the melting temperature and results in a fine dispersion of aluminum rich regions in the Greenkote structure. The overall thickness of the Zn-Fe diffusion layer continues to thicken until the zinc powder is consumed — completing the process.
Greenkote process technology — Final Result:
Greenkote’s thermal diffusion coating process forms a protective layer that is physically diffused into the substrate. Metallurgically bonded, it cannot be easily separated by physical or environmental forces. In addition, the aluminum-rich inclusions dispersed on the surface serve to heal any micro fissures, cracks or porosity in the coating, while the zinc provides a sacrificial barrier against corrosion. The result is a highly uniform, conformal coating that gives exceptional protection against corrosion as well as enhanced durability and other performance advantages. The natural micro-roughness of the Greenkote surface gives it superior adhesion qualities for metal topcoats as well as for paints.
Innovative anti-corrosion technology
The primary cause of corrosion in basic thermo-mechanical sherardizing processes is the electrochemical reaction between the substrate (consisting mainly of iron) and the intermetallic Zn-Fe coating. To counter this effect, the Greenkote process introduces sacrificial phases to protect Zn-Fe intermetallics from direct electrochemical reaction with the substrate material. Greenkote’s aluminum-rich surface inclusions formed from Al-Fe intermetallics and Zn-Al alloys serve as such sacrificial phases. Because Al-Fe intermetallics and Zn-Al alloys are more chemically active than intermetallic Fe-Zn, they are not as likely to experience self-passivation as is aluminum. In Zn-Al alloys and Al-Fe intermetallics a continuous passivation film of aluminum hydroxides is not formed.
In addition, the products of corrosion of the aluminum-rich phases possess low solubility in water. They precipitate on coating defects (cracks, pores, etc.) and fill them, thereby slowing down the corrosion rate considerably. Hence, the benefit of the aluminum-rich dispersions continues, even after the sacrificed phase is consumed, giving the coating the ability to heal or self-repair in operation.
Enhanced alloy chemistry and unique coating structure provide Greenkote-processed components with uniform, high-density, damage-resistant surface protection. These are key reasons for Greenkote’s higher corrosion resistance compared with other types of Zn-Fe coatings.
Greenkote® and the Greenkote logo are registered trademarks of Greenkote PLC.